
New to the world of three-dimensional (3D) biology, or testing out a new material with your Allevi bioprinter? This protocol is part of a series to help you learn how to design experiments and easily build with life! Before you begin bioprinting, you need to make sure you have proper 3D controls.
Typical two-dimensional (2D) cell culture controls are often not completely accurate comparisons to 3D printed constructs. Thin-film 3D controls help provide better insight by allowing you to control for more variables. 3D controls can be pipetted or bioprinted. Pipetted thin films are ideal for an encapsulation study to isolate the effect of the material on the cells without subjecting them to bioprinting. Bioprinted thin films are useful for isolating how the cells react to undergoing extrusion.
What’s a Thin-Film 3D Control, and how do I make it?
Great question! A 3D thin film is a small volume of your material, ideally no thicker than 200μm, that can be used to analyze cell viability and compare with bioprinted samples.
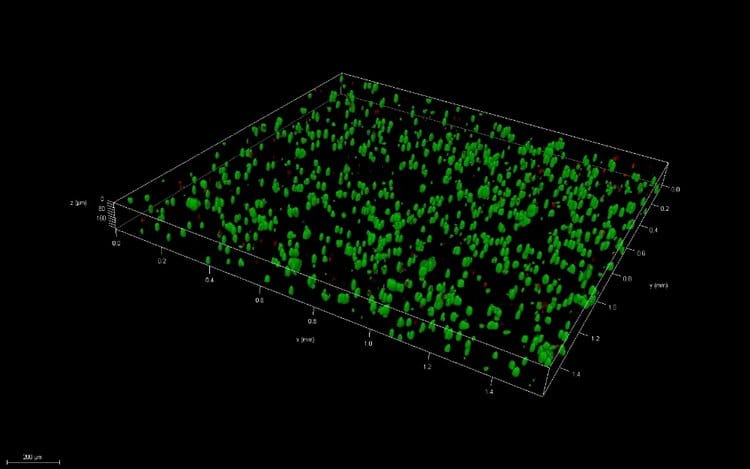
Pipetted thin films
We create many of our pipetted thin films by simply pipetting small volumes of the material (5 – 10 μl) with encapsulated cells. Sometimes, we use a variety of methods to flatten the material after pipetting to ensure the height of the thin film is no thicker than 200 μm. Why does this thickness matter? In the body, cells can’t survive farther than this distance from blood vessels, or their source of nutrients. Ensuring the thickness is no larger than this distance removes the potential of decreased viability due to the geometry of the structures.
Bioprinted thin films
When bioprinting, we create these thin films by extruding small volumes of the material (5 – 10 μl) with encapsulated cells for a set amount of time. This can be accomplished by utilizing a custom G-code file like the one below:
; Sample Code – I can label my code and create comments by writing text after a semicolon
G1 Z0.1 ; Set this to your desired layer height
T0 ; select left extruder for this print (T0 = E1, T1 = E2, etc)
G92 E0 ; Reset extrusion
G1 X0 Y0 ; Move extruder to (0,0)
G1 E0.2 ; Start extruding
G4 S2 ; extrude for 2 seconds (change the number 2 to desired amount of extrusion time)
G92 E0 ; Stop extrudingThe time needed for extrusion to create a specific volume can be determined through the volume test, which is described in more detail in our post about characterizing print parameters for a new bioink. You can use these thin films to analyze the effects of the printing process on construct viability. If you are not able to achieve sufficiently high cell viability in your bioprinted thin films, for example, you’ll know there is an issue with your bioprinting parameters (such as pressure or needle type).
Setting Up Your Experiment
Determine Your Groups
What do you want to test in this study? Consider potential variables you can test that might be affected by the printing process. What pressure do you plan to print at? What needle type are you going to use? How long will your print take? At a minimum, you should have three groups: a 2D control, a pipetted thin-film 3D control, and a 3D bioprinted thin-film experimental group.
For example, you can run an initial bioprint to analyze the effects of different pressures. See example groups with our material Sodium Alginate:
| Control | Bioink | Needle | Pressure (PSI) |
|---|---|---|---|
| 2D cell culture | N/A | N/A | N/A |
| Pipetted thin-film | 2% (w/v) alginate | N/A | N/A |
| Bioprinted thin-film | 2% (w/v) alginate | 0.25″ straight 27G | 5 |
| Bioprinted thin-film | 2% (w/v) alginate | 0.25″ straight 27G | 10 |
Alternatively, you may want to test different needle types. That study may contain groups such as:
| Control | Bioink | Needle | Pressure (PSI) |
|---|---|---|---|
| 2D cell culture | N/A | N/A | N/A |
| Pipetted thin film | 2% (w/v) alginate | N/A | N/A |
| Bioprinted thin-film | 2% (w/v) alginate | 0.25″ straight 27G | 10 |
| Bioprinted thin-film | 2% (w/v) alginate | 0.25″ straight 30G | 10 |
Determine Analytic Tests and Timepoints
How will you analyze your results? Your material and what variables you are testing will largely determine what assays are best to use. When testing for viability, Live/Dead staining is always a good qualitative control. We have successfully used Live/Dead staining (Calcein-AM, Ethidium Homodimer) with all of our matrix reagents.
Some other common assays used to analyze 3D samples include the AlamarBlue Assay and ATP Cell Titer Glo 3D Assay. However, these assays sometimes require you to break apart your samples, especially if the assay solution does not effectively permeate your specific material.
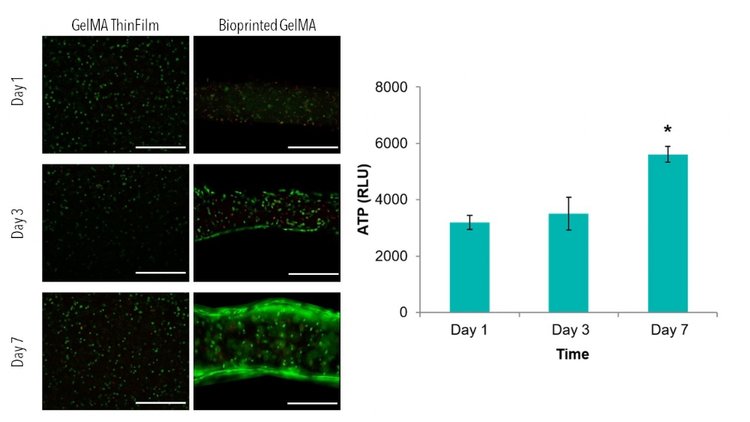
If you plan to eventually test function, other assays or testing methods may be necessary. Finally, depending again on what variables you plan to analyze, you will have to decide what time-points to use (When analyzing viability, we often complete a 7-day study with time-points at days 1, 3, and 7).
Determine Necessary Cell Number and Material Needed for Study
Now you just need to plan how much material and how many cells you will need for your experiment! Once you complete this study, you can then use your bioprinted thin-film 3D controls in future bioprinting experiments!
Examples
Check out some of our bioreports for results and methods with 3D thin films.

